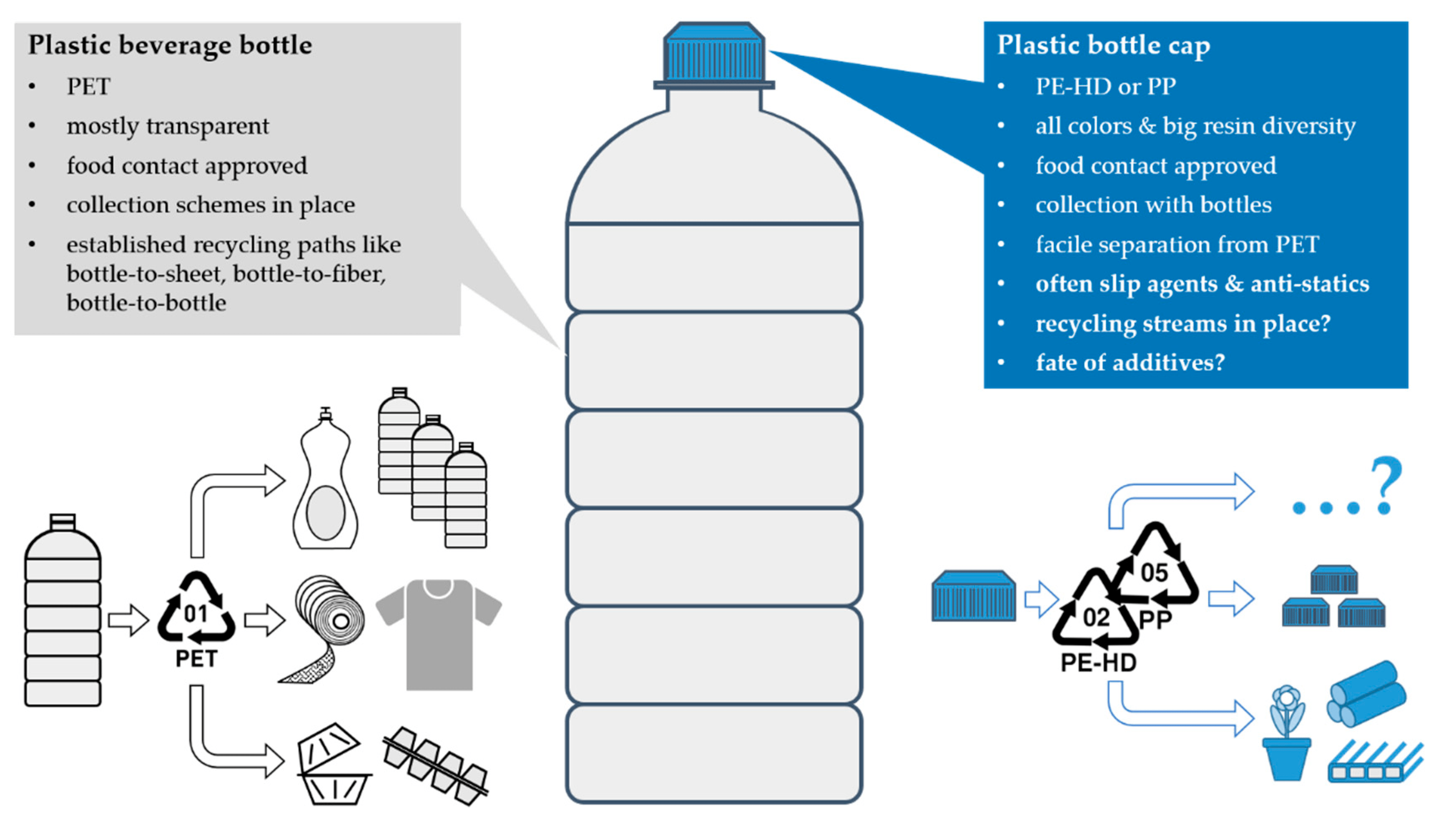
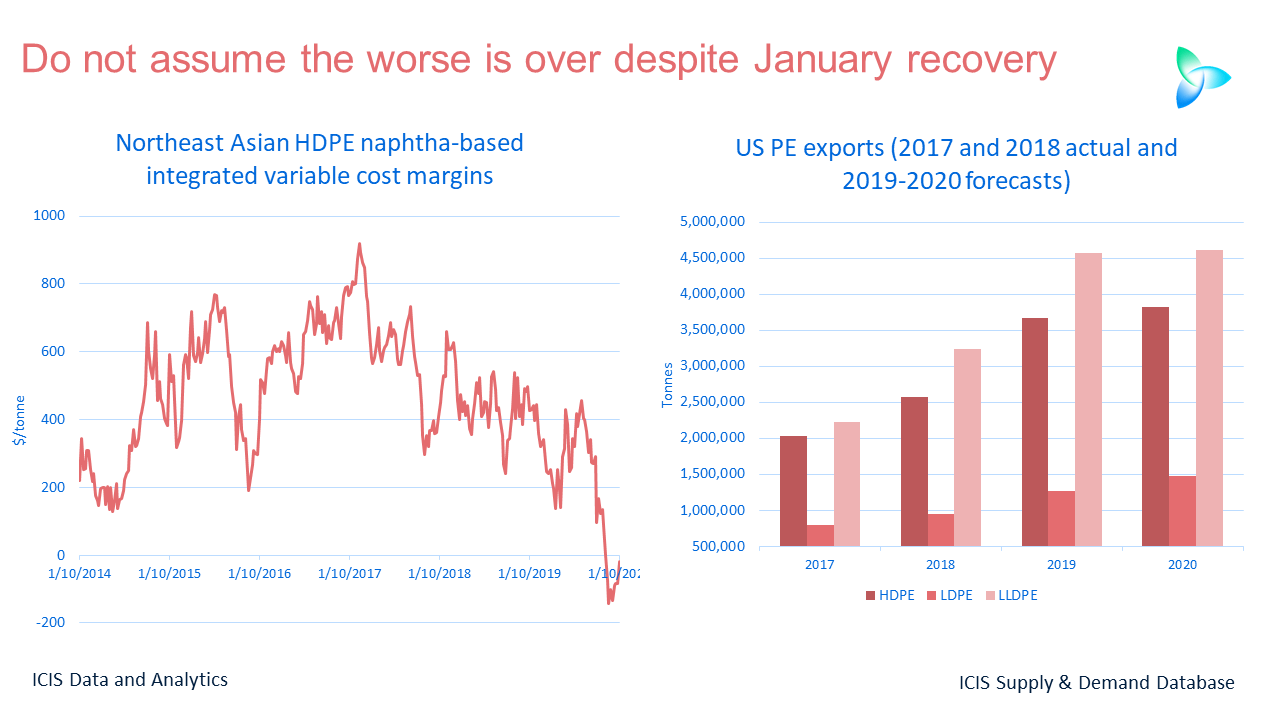
Introduction: Organic chemistry, often dubbed the “chemistry of life,” unravels the mysteries of molecules primarily composed of carbon. It’s a realm where atoms dance to form bonds, leading to the myriad substances that make up our world, from the DNA in our cells to the gasoline that fuels our cars. Let’s embark on a journey to understand the basics of this fascinating field.
1. What is Organic Chemistry?
Organic chemistry studies compounds containing carbon, excluding a few exceptions like carbonates and cyanides. Given carbon’s unique ability to form strong covalent bonds with other carbon atoms, millions of organic compounds exist, each with its distinct properties.
2. The Significance of Carbon:
Carbon’s versatility is rooted in its atomic structure.
- Tetravalency: Carbon has four electrons in its outermost shell and requires four more to achieve stability. This enables it to form four covalent bonds with other atoms.
- Catena Formation: Carbon’s ability to link with other carbon atoms creates long chains or rings, known as catenation.
3. Hydrocarbons: The Building Blocks
The simplest organic compounds are hydrocarbons, consisting solely of carbon and hydrogen.
- Alkanes: Single bonded compounds like methane (CH4).
- Alkenes: Contain at least one double bond, such as ethene (C2H4).
- Alkynes: Feature one or more triple bonds, like ethyne (C2H2).
4. Functional Groups: Character-Defining Entities
These are specific groups of atoms within molecules responsible for characteristic reactions. Examples include:
- Alcohols: Containing the -OH group.
- Carboxylic Acids: Featuring the -COOH group.
- Amines: Comprising the -NH2 group.
5. Isomerism: Same Formula, Different Structures
Isomers are compounds with the same molecular formula but different structural arrangements. This phenomenon showcases carbon compounds’ diversity.
- Structural Isomers: Differ in the connectivity of atoms.
- Geometric Isomers: Differ in spatial orientation due to restricted rotation, often seen in alkenes.
6. The Magic Behind Reactions:
Organic chemistry is rife with reactions transforming one compound into another.
- Substitution Reactions: An atom or group in a molecule gets replaced by another atom or group.
- Addition Reactions: Atoms or groups are added to double or triple bonds, converting them to single bonds.
- Elimination Reactions: Atoms or groups are removed from a molecule, leading to the formation of double or triple bonds.
7. Organic Chemistry in Daily Life:
From the food we consume to the medicines that heal, organic compounds are omnipresent.
- Pharmaceuticals: Organic reactions are employed to synthesize a plethora of drugs.
- Plastics: Polymers, long chains of repeating units, form the basis of the plastic industry.
- Cosmetics: From the fragrances we adore to the creams we apply, organic chemistry plays a pivotal role.
8. Challenges and Frontiers:
Organic chemistry continually evolves, responding to global challenges.
- Green Chemistry: In response to environmental concerns, there’s a push to develop sustainable methods that minimize waste and toxicity.
- Drug Discovery: With evolving diseases, the race is on for newer, effective medications.
- Synthesis of Complex Molecules: As our understanding deepens, chemists strive to build increasingly intricate structures, testing the limits of what’s possible.
Conclusion:
Organic chemistry, with its vast array of molecules and reactions, is a testament to the beauty and complexity of the natural world. It’s a realm where science meets art, where understanding the intricate ballet of atoms can lead to innovations that change the world.
Tags: #OrganicChemistry, #MagicOfMolecules, #CarbonCompounds, #Hydrocarbons, #FunctionalGroups, #ChemicalReactions, #MolecularMagic