Acid-base chemistry is a fundamental branch of chemistry that plays a crucial role in our daily lives and in various scientific disciplines. From the sour taste of citrus fruits to the pH of household cleaners, acid-base reactions are all around us. In this article, we will explore the basics of acid-base chemistry, including definitions, properties, and examples of common acids and bases.
Acids and Bases Defined
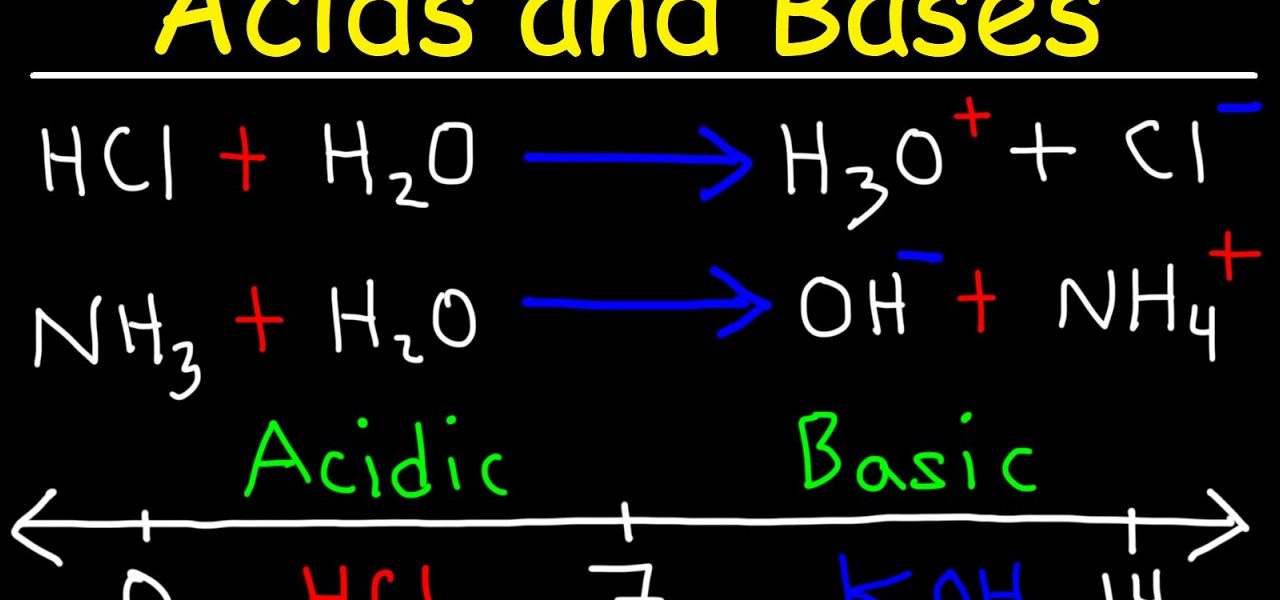
- Acids: Acids are substances that can donate a proton (H+) to another substance. They are characterized by a sour taste, the ability to turn blue litmus paper red, and a pH value less than 7. Common examples include hydrochloric acid (HCl) and acetic acid (found in vinegar).
- Bases: Bases, also known as alkaline substances, are compounds that can accept a proton (H+). They have a bitter taste, a slippery feel, and a pH value greater than 7. Examples of bases include sodium hydroxide (NaOH) and ammonia (NH3).
The pH Scale
- pH Measurement: The pH scale is used to measure the acidity or alkalinity of a solution. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH less than 7 are acidic, while those with a pH greater than 7 are basic.
- pH Indicators: Indicators like litmus paper, phenolphthalein, and pH meters are used to determine the pH of a solution.
Acid-Base Reactions
- Neutralization: When an acid and a base react, they undergo a neutralization reaction, resulting in the formation of water and a salt. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), it forms water (H2O) and sodium chloride (NaCl).
- Hydrogen Ion Transfer: In acid-base reactions, the transfer of hydrogen ions (protons) is fundamental. The acid donates a proton, and the base accepts it.
Common Acids and Bases
- Strong Acids: Strong acids completely dissociate in water, producing a high concentration of hydrogen ions. Examples include sulfuric acid (H2SO4) and nitric acid (HNO3).
- Weak Acids: Weak acids only partially dissociate in water, resulting in a lower concentration of hydrogen ions. Acetic acid (CH3COOH) is an example of a weak acid.
- Strong Bases: Strong bases readily accept protons and dissociate completely in water. Sodium hydroxide (NaOH) and potassium hydroxide (KOH) are strong bases.
- Weak Bases: Weak bases only partially accept protons and have lower dissociation constants. Ammonia (NH3) is a weak base.
Applications of Acid-Base Chemistry
- Antacids: Antacids like calcium carbonate (CaCO3) neutralize excess stomach acid, relieving heartburn and indigestion.
- Cleaning Products: Many household cleaning products are basic and effective at removing stains and grease.
- Food Preservation: Acidic substances like citric acid and vinegar are used to preserve food by lowering the pH and inhibiting the growth of microorganisms.
- Environmental Chemistry: Acid rain, caused by sulfur dioxide (SO2) and nitrogen oxides (NOx) in the atmosphere, has detrimental effects on ecosystems and infrastructure.
Buffers and pH Regulation
- Buffers: Buffers are solutions that resist changes in pH when an acid or base is added. They are essential for maintaining stable pH levels in biological systems.
- Biological Systems: In living organisms, pH regulation is crucial for maintaining cellular functions. Enzymes, for example, are sensitive to pH changes.
Conclusion
Acid-base chemistry is a foundational concept with broad implications in various fields, from chemistry and biology to environmental science and medicine. Understanding the basics of acids, bases, and pH allows us to comprehend a wide range of natural phenomena and provides a foundation for further exploration of this essential branch of chemistry. Whether in the laboratory, in daily life, or in critical applications like healthcare and environmental protection, a grasp of acid-base chemistry is indispensable.