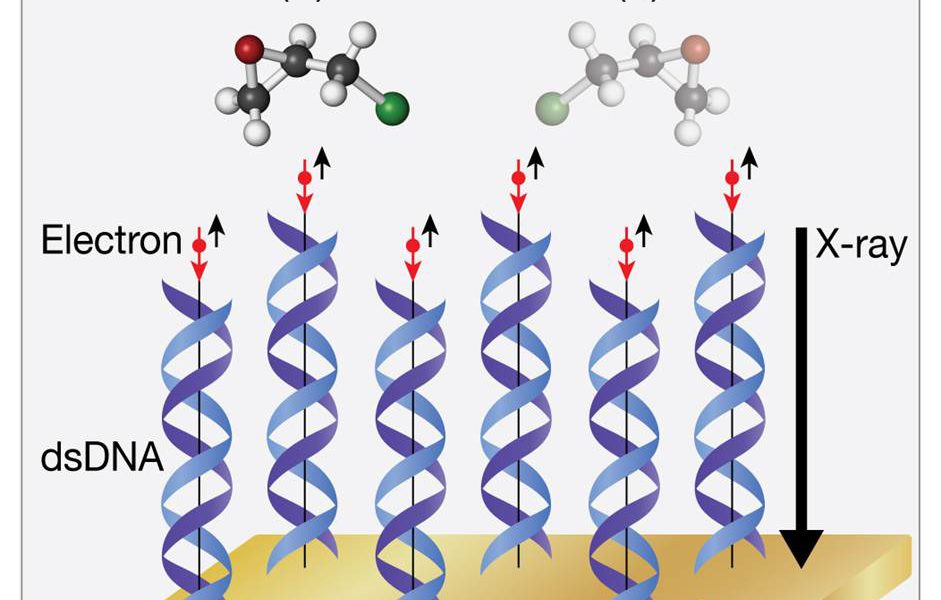
Molecules, much like human hands, can be mirror images of each other. The phenomenon, known as chirality, plays a critical role in chemistry and, by extension, life itself. The term ‘chiral’ originates from the Greek word for ‘hand’, and it offers a perfect analogy: just as left and right hands are mirror images but not superimposable, so too are certain molecules. But why does nature exhibit this bias, and how does it impact life?
1. Introduction to Chirality:
In the microscopic realm, certain molecules exist in two forms that are mirror images of each other. These forms, called enantiomers, are much like left and right hands: identical in structure but opposite in orientation. A molecule possessing this characteristic is said to be chiral.
2. Recognizing Chiral Molecules:
A molecule is chiral if it has an asymmetrical center, often a carbon atom bonded to four different atoms or groups. This central atom is called a chiral or stereocenter. The presence of a stereocenter often leads to the formation of non-superimposable mirror image forms.
3. Biological Significance of Chirality:
Many biological molecules, including amino acids and sugars, are chiral. The fascinating fact is that life, especially as we know it on Earth, often prefers one enantiomer over the other. For instance, amino acids in proteins are almost exclusively left-handed, while sugars in DNA and RNA are right-handed.
4. Chirality in Drugs and Medicine:
The handedness of molecules can significantly affect their biological activity. One enantiomer might exhibit therapeutic properties, while its mirror image might be inactive or even harmful. Thalidomide, a drug introduced in the 1950s, serves as a poignant example. One enantiomer was effective as a sedative and anti-nauseant, but its mirror image caused severe birth defects.
5. Detecting Chirality:
When plane-polarized light passes through a solution of a chiral compound, it rotates either to the left or right. This property, known as optical activity, helps chemists determine the presence and concentration of chiral molecules.
6. Nature’s Preference:
The question remains: why does nature favor one enantiomer over another? Various theories exist, from chance events in prebiotic chemistry to the influence of cosmic forces. Some scientists speculate that polarized light from neutron stars might have preferentially destroyed one enantiomer, leading to an excess of the other. However, this remains a topic of ongoing research.
7. Chirality in Extraterrestrial Life:
If we encounter life beyond Earth, will it exhibit the same chiral preferences? Some scientists theorize that extraterrestrial life might utilize the opposite enantiomers, leading to ‘mirror life.’ While still in the realm of speculation, this has implications for astrobiology and the search for life beyond our planet.
8. The Chiral Pool in Prebiotic Chemistry:
The origin of life’s chiral preference remains one of chemistry’s great mysteries. Before life began, both enantiomers would have been present in equal amounts, a racemic mixture. Yet, life chose one over the other. Understanding this ‘chiral induction’ could offer clues to the origins of life itself.
9. Artificial Creation of Chirality:
In laboratories, chemists can now synthesize both enantiomers of a molecule. Using chiral catalysts or optically active starting materials, they can control the orientation of the final product, leading to breakthroughs in pharmaceuticals and material science.
10. Conclusion:
Chirality, or molecular handedness, is a subtle yet profound concept. Its implications touch everything, from the proteins in our cells to potential life on distant worlds. As we continue to unravel the mysteries of chirality, we gain deeper insights into the intricate dance of molecules that underpin the very fabric of life.
Tags:
#Chirality #MolecularHandedness #Enantiomers #OpticalActivity #ChiralLife #AsymmetryInNature #Biochemistry #Astrobiology