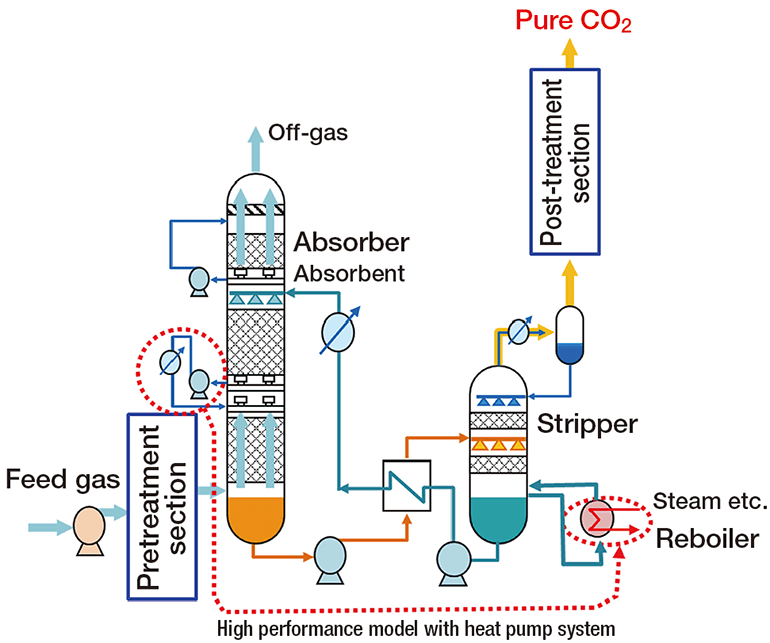
Photography, an art form and a science, owes much of its existence to the intricate world of chemistry. From the early days of daguerreotypes to the modern digital cameras, the evolution of photography has been significantly influenced by chemical discoveries and innovations. This 1000-word article delves into the fascinating chemistry behind photography, exploring how chemical reactions have captured and preserved our visual memories.
1. Introduction to the Chemistry of Photography
Photography is fundamentally a chemical process where light interacts with sensitive materials to create images. This process involves a series of chemical reactions, which are initiated by light and developed through various chemical solutions. Understanding these chemical principles is crucial to appreciating the magic of photography.
2. The Early Days: Silver-Based Photography
The earliest photographic processes, such as daguerreotypes and wet plate collodion processes, relied on silver salts due to their light-sensitive nature.
Silver Halides in Photography
Silver halides, primarily silver bromide (AgBr), silver chloride (AgCl), and silver iodide (AgI), are key components in photographic film. These compounds are sensitive to light; when exposed to photons, they undergo a photochemical reaction that forms a latent image.
The Latent Image Formation
The latent image is the invisible image formed on the film when it is exposed to light. During exposure, photons cause the silver ions in the silver halide crystals to reduce to metallic silver. This forms the latent image, which is then developed to become visible.
3. The Development Process
Developing a photograph involves converting the latent image into a visible one. This is achieved through a series of chemical baths.
Developing Solution
The developer solution contains reducing agents, typically based on compounds like hydroquinone or phenidone. These agents amplify the latent image by converting the exposed silver halide crystals entirely into metallic silver, revealing the image.
Stopping the Development
After development, the film is placed in a stop bath, usually an acidic solution, to halt the development process immediately. This prevents overdevelopment of the image.
Fixing the Image
The fixing stage is crucial to make the image permanent. A fixer solution, often containing compounds like sodium thiosulfate, removes the unexposed silver halide crystals, leaving only the developed image on the film.
4. The Role of Light in Photography
Light is the catalyst for the chemical reactions in photography. The intensity and duration of light exposure determine how the silver halides react, affecting the contrast and brightness of the final image.
5. Color Photography: Adding Colors to Memories
The advent of color photography added complexity to the chemistry involved.
Color Film Structure
Color films have multiple layers of emulsion, each sensitive to different colors of light (typically red, green, and blue). Each layer contains different color-forming chemicals, or couplers, which react during development to produce the colors.
Color Development Process
In color photography, the development process involves several steps, including color development, bleaching, fixing, and sometimes stabilizing. The color development not only reduces the exposed silver halides but also forms colored dyes in the emulsion layers, creating the color image.
6. Polaroid Photography: Instant Chemistry
Polaroid or instant photography brought a unique chemistry. It combines the negative and positive layers along with the developing chemicals in a single film pack. When the photo is taken, the developer is spread across the film, initiating the development process immediately and resulting in an instant photograph.
7. Digital Photography: A Shift from Chemical to Electronic
With the advent of digital photography, the role of chemistry has shifted. Digital cameras use electronic sensors to capture images, converting light into electronic signals, which are then processed and stored digitally. While the fundamental principle of capturing light remains, the chemical processes have been replaced by electronic ones.
8. Photographic Paper and Printing
Photographic printing involves projecting the negative image onto a photosensitive paper, followed by similar chemical processes of developing, stopping, and fixing to create a physical print.
9. The Environmental Impact of Photographic Chemicals
The chemicals used in traditional photography, especially in development and fixing, can have environmental impacts. Proper disposal and recycling of these chemicals are important to minimize ecological damage.
10. Preservation and Degradation of Photographs
The chemical nature of photographs makes them susceptible to degradation over time. Factors like light exposure, humidity, and temperature can affect the stability of the chemicals in photographs, leading to fading or color shifts.
11. Future of Photography: Sustainable Practices and Innovations
As technology advances, photography continues to evolve. The future may see more sustainable and environmentally friendly photographic processes, along with continued innovations in digital imaging technologies.
Conclusion
The chemistry behind photography is a testament to the remarkable ways in which science and art intersect. From the intricate dance of silver halide crystals in response to light to the electronic sensors of digital cameras, the journey of photography is rich with chemical innovation. Understanding these chemical processes not only enhances our appreciation of the art of photography but also highlights the important role of chemistry in capturing and preserving our visual world. As photography continues to evolve, the chemistry behind it remains a fundamental part of its magic, reminding us of the enduring bond between science and art.