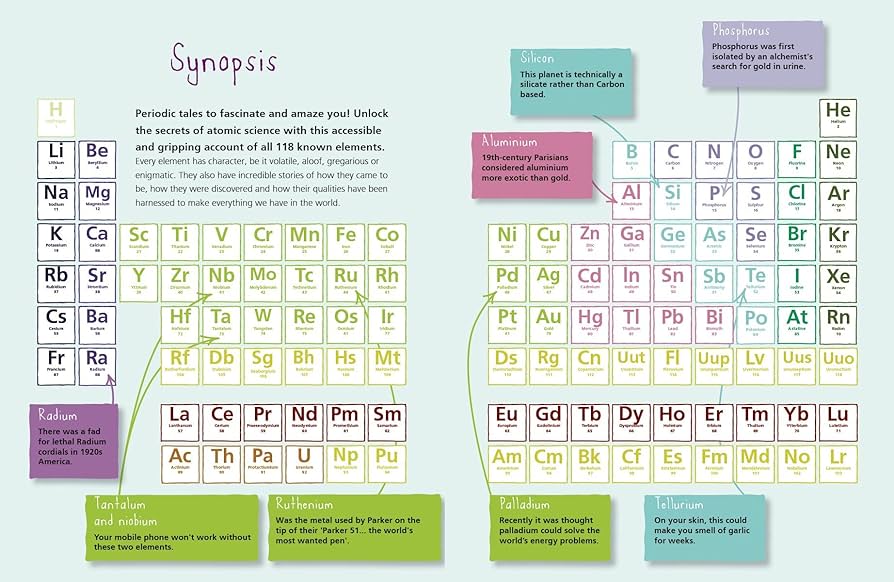
The Periodic Table stands as a visual masterpiece, organizing the building blocks of matter into a systematic and danatoto array. This article embarks on a scientific journey, unraveling the secrets encoded in the Periodic Table, exploring its history, significance, and the profound impact it has had on the understanding of the fundamental elements.
The Historical Tapestry:
- Mendeleev’s Vision: In 1869, Russian chemist Dmitri Mendeleev conceived the first version of the Periodic Table, arranging elements by atomic mass and predicting the existence of undiscovered elements. His foresight laid the foundation for the modern Periodic Table.
- Elemental Discoveries: The Periodic Table evolved alongside discoveries of new elements. From the isolation of hydrogen by Henry Cavendish to the synthesis of synthetic elements, each addition expanded the table, offering new insights into the elemental realm.
Organizing the Elements:
- Periods and Groups: The table’s structure is based on periods (rows) and groups (columns). Elements in the same group share similar chemical properties, while periods signify the filling of electron shells, dictating atomic behavior.
- Metals, Nonmetals, and Metalloids: The Periodic Table neatly classifies elements into metals, nonmetals, and metalloids based on their properties. This classification aids in predicting an element’s behavior in chemical reactions.
Atomic Properties and Trends:
- Atomic Radius and Electronegativity: The Periodic Table illustrates trends in atomic radius and electronegativity. Moving across a period, atomic radius decreases, while electronegativity generally increases. Down a group, atomic radius expands, and electronegativity decreases.
- Valence Electrons and Reactivity: Valence electrons, the outermost electrons of an atom, dictate reactivity. Elements in the same group share the same number of valence electrons, influencing their chemical behaviors and potential reactions.
The Impact on Chemistry:
- Predicting Chemical Behavior: One of the Periodic Table’s triumphs is its ability to predict the chemical behavior of elements. By understanding an element’s position, scientists can anticipate its reactions, forming the basis for countless chemical advancements.
- Noble Gases and Inertness: The discovery of noble gases, occupying Group 18, revealed a class of elements with minimal chemical reactivity. Helium, neon, argon, krypton, xenon, and radon showcase inertness, finding applications in various technological fields.
Technological Applications:
- Catalysts and Transition Metals: Transition metals, found in the central block of the Periodic Table, serve as vital catalysts in industrial processes. Their unique electron configurations make them instrumental in facilitating chemical reactions.
- Semiconductors and Metalloids: Metalloids, situated on the border between metals and nonmetals, exhibit semiconductor properties. Elements like silicon and germanium have become foundational materials in the electronics industry.
Synthetic Elements and Beyond:
- Beyond Natural Elements: The discovery of synthetic elements, created through advanced laboratory processes, expands the Periodic Table beyond naturally occurring elements. Elements like technetium, promethium, and the superheavy elements push the boundaries of our understanding.
- The Quest for Stability: The search for new elements aims not only to expand the table but also to discover superheavy elements with increased stability. Such elements could unlock novel possibilities in materials science and nuclear technology.
Educational Tool and Scientific Guide:
- Teaching Fundamental Concepts: The Periodic Table serves as an educational cornerstone, teaching students fundamental concepts in chemistry. Its visual representation aids in understanding relationships between elements and their properties.
- Navigating Elemental Relationships: For scientists, the Periodic Table is an indispensable tool. Its arrangement facilitates the exploration of elemental relationships, guiding research, and providing a roadmap for the synthesis of new compounds.