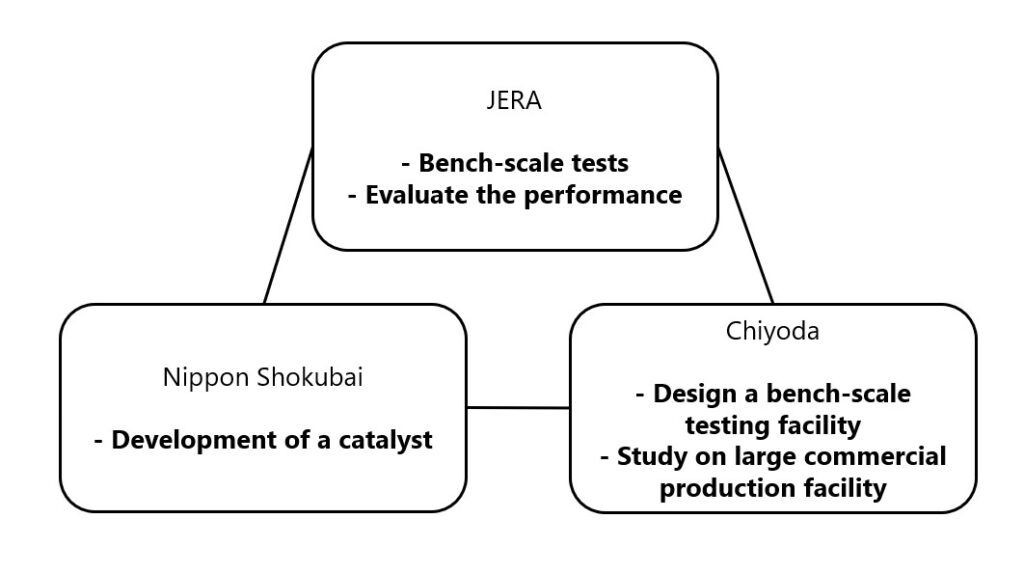
Chemical reactions are the heart of chemistry, occurring all around us, from the food we eat to the air we breathe. They are the processes that transform matter, either through the formation of new chemical bonds or the breaking of existing ones. This 1000-word article delves into the fascinating world of chemical reactions, exploring how they shape our world and impact our daily lives.
Introduction to Chemical Reactions
At its core, a chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Classified into several types based on the nature of the transformations, these reactions are the fundamental processes in chemistry that enable the creation of new substances with distinct properties.
Understanding the Basics of Chemical Reactions
Reactants and Products
In a chemical reaction, the starting substances are called reactants, and the substances formed are known as products. The transformation from reactants to products involves the rearrangement of atoms and the making or breaking of chemical bonds.
The Chemical Equation
Chemical reactions are represented by chemical equations, which express the reactants and products along with their quantitative relationships. Balancing these equations is crucial for representing the conservation of mass in a reaction.
Types of Chemical Reactions
Chemical reactions can be broadly classified into several types, each with unique characteristics:
- Synthesis (Combination) Reactions: Two or more substances combine to form a single product.
- Decomposition Reactions: A single compound breaks down into two or more simpler substances.
- Single Replacement Reactions: One element replaces another in a compound.
- Double Replacement Reactions: The ions of two compounds exchange places in an aqueous solution to form two new compounds.
- Combustion Reactions: Substances combine with oxygen, releasing energy, typically as heat and light.
Energy in Chemical Reactions
The concept of energy is central to understanding chemical reactions. Reactions can be exothermic (releasing energy) or endothermic (absorbing energy). The energy change in a reaction is determined by the differences in bond energies of the reactants and products.
Activation Energy
Activation energy is the minimum energy required to initiate a chemical reaction. Catalysts are substances that lower the activation energy, speeding up the reaction without being consumed in the process.
Chemical Reactions in Everyday Life
Chemical reactions are not just laboratory phenomena; they are an integral part of everyday life:
- Cooking: Cooking involves various chemical reactions, including the Maillard reaction, which gives cooked food its flavor and color.
- Metabolism: The body’s metabolism is a series of chemical reactions essential for life, converting food into energy.
- Cleaning: Cleaning agents work through chemical reactions, where substances like detergents break down and remove dirt and stains.
The Role of Chemical Reactions in Industry
Industrial processes are heavily reliant on chemical reactions. The manufacturing of materials like plastics, metals, and pharmaceuticals involves complex chemical reactions. The Haber process for ammonia synthesis and the contact process for sulfuric acid production are prime examples of chemical reactions used in industry.
Chemical Reactions and the Environment
Chemical reactions play a significant role in environmental processes:
- Photosynthesis: The process by which plants convert carbon dioxide and water into glucose and oxygen is a fundamental chemical reaction driving life on Earth.
- Atmospheric Reactions: Chemical reactions in the atmosphere are responsible for phenomena like the formation of ozone and the greenhouse effect.
The Impact of Chemical Reactions in Medicine
In the field of medicine, chemical reactions are crucial for the development and action of drugs. The body’s response to medication involves complex biochemical reactions. Additionally, synthetic chemistry is used to create a wide range of pharmaceuticals.
Chemical Reactions and Energy Production
Chemical reactions are at the heart of energy production. Combustion reactions power vehicles and generate electricity, while electrochemical reactions are the basis of batteries and fuel cells.
The Science of Reaction Rates: Chemical Kinetics
Chemical kinetics is the study of reaction rates, understanding how various factors like concentration, temperature, and catalysts affect the speed of reactions. This field is essential for controlling and optimizing reactions in industrial processes.
Innovative Research in Chemical Reactions
Ongoing research in chemical reactions focuses on developing more efficient, sustainable, and environmentally friendly processes. This includes the study of green chemistry practices, the development of new catalysts, and the exploration of alternative energy sources.
Safety and Chemical Reactions
Safety is a critical aspect when dealing with chemical reactions, especially in industrial and laboratory settings. Proper handling, storage, and disposal of chemicals and understanding the potential hazards of reactions are essential for preventing accidents and environmental damage.
Conclusion
Chemical reactions are fundamental processes that drive the natural world and human-made technologies. From the simplest everyday activities to the most advanced scientific endeavors, chemical reactions are integral to our existence and progress. Understanding these reactions not only enhances our appreciation of the world around us but also enables us to harness their power for various applications. As we continue to explore and manipulate chemical reactions, they will undoubtedly play a crucial role in addressing some of the most pressing challenges of our time, from energy production to environmental protection and the advancement of medicine.