Essential oils, the highly concentrated extracts from plants, have fascinated humans for centuries. Used in aromatherapy, medicine, and cosmetics, these oils capture the essence of plants, offering a window into the complex chemistry of nature. This 1000-word article delves into the chemistry behind essential oils, exploring how they are formed, their components, and their myriad uses and benefits.
1. Introduction to Essential Oils
Essential oils are volatile, aromatic compounds extracted from various parts of plants, including flowers, leaves, bark, roots, and fruits. Traditionally used in aromatherapy and alternative medicine, these oils have gained popularity for their therapeutic properties and are now widely used in various industries.
2. The Extraction Process
The extraction of essential oils is a delicate process that requires preserving the integrity of the aromatic compounds. The most common methods include:
- Steam Distillation: The most prevalent method where steam is used to vaporize the volatile compounds, which are then condensed back into a liquid form.
- Cold Pressing: Primarily used for citrus oils, this mechanical method involves pressing the fruit to extract the oils.
- Solvent Extraction: Used for delicate flowers, this method uses solvents to extract the oils, which is then removed, leaving behind the pure oil.
3. The Complex Chemistry of Essential Oils
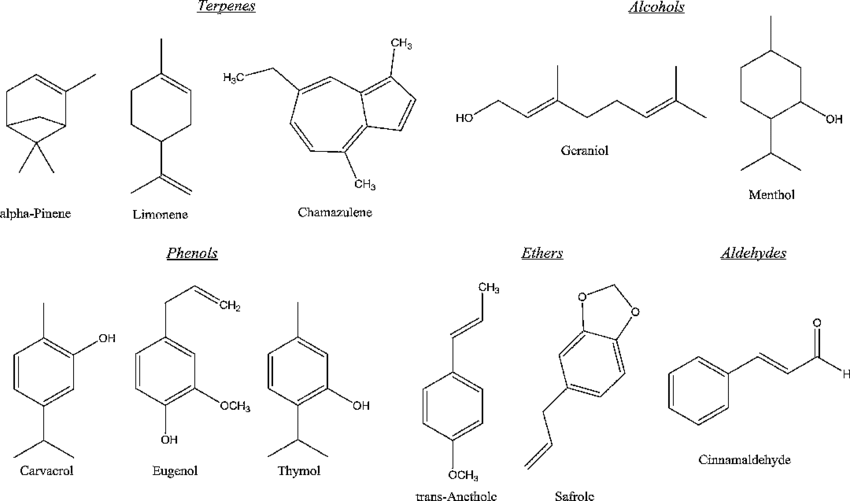
Essential oils are composed of a complex mixture of chemical compounds, primarily terpenes, terpenoids, and phenolics, which give them their characteristic aromas and therapeutic properties.
Terpenes and Terpenoids
Terpenes are simple hydrocarbons and the most abundant compounds in essential oils. Examples include pinene in pine oil and limonene in citrus oils. Terpenoids are modified terpenes that contain additional functional groups and are responsible for the vast array of scents and flavors in essential oils.
Phenolic Compounds
Phenolic compounds in essential oils, such as eugenol in clove oil and thymol in thyme oil, are known for their antiseptic and anti-inflammatory properties. These compounds contribute significantly to the therapeutic effects of essential oils.
4. Therapeutic Properties and Uses
The unique chemical composition of essential oils lends them a variety of therapeutic properties:
- Antimicrobial Activity: Many essential oils have been shown to possess antibacterial, antifungal, and antiviral properties. For instance, tea tree oil is widely known for its ability to combat infections and boost immunity.
- Aromatherapy and Psychological Effects: Essential oils like lavender and chamomile are used in aromatherapy for their calming and relaxing effects, helping to reduce stress and improve sleep quality.
- Anti-inflammatory and Analgesic Properties: Some oils, such as eucalyptus and peppermint oil, are known for their anti-inflammatory and pain-relieving properties, making them popular in treating muscle aches and arthritis.
5. Essential Oils in Cosmetics and Skincare
In the cosmetics industry, essential oils are prized for their aromatic properties and skin benefits. Oils like rosehip and frankincense are incorporated into skincare products for their anti-aging and moisturizing properties, while tea tree and lavender oils are used for their ability to treat acne and soothe skin irritation.
6. Role in Food and Beverage Industry
Essential oils also find extensive use in the food and beverage industry as flavoring agents. Citrus oils, mint, and cinnamon oil are commonly used to impart distinct flavors to food and drinks.
7. The Science Behind Aromatherapy
Aromatherapy involves the use of essential oils for therapeutic purposes. The olfactory system plays a key role here – when inhaled, the molecules in essential oils interact with the receptors in the nose, triggering responses that affect the limbic system in the brain, which is associated with emotions, heart rate, breathing, and memory.
8. Safety and Precautions
While essential oils offer numerous benefits, they must be used with caution:
- Concentration and Dilution: Essential oils are highly concentrated and should be diluted with a carrier oil before topical application to avoid skin irritation.
- Ingestion Risks: Most essential oils are not safe for ingestion and can be toxic if swallowed.
- Allergic Reactions and Sensitivity: Patch tests are recommended before using essential oils to check for allergic reactions.
9. Scientific Research and Studies
The field of aromatherapy and essential oils is a growing area of scientific research. Studies are continuously being conducted to better understand their pharmacological properties, mechanisms of action, and potential therapeutic uses.
10. Sustainability and Ethical Sourcing
The increasing demand for essential oils raises concerns about sustainability and ethical sourcing. Overharvesting and exploitation of resources can lead to environmental damage and loss of biodiversity.