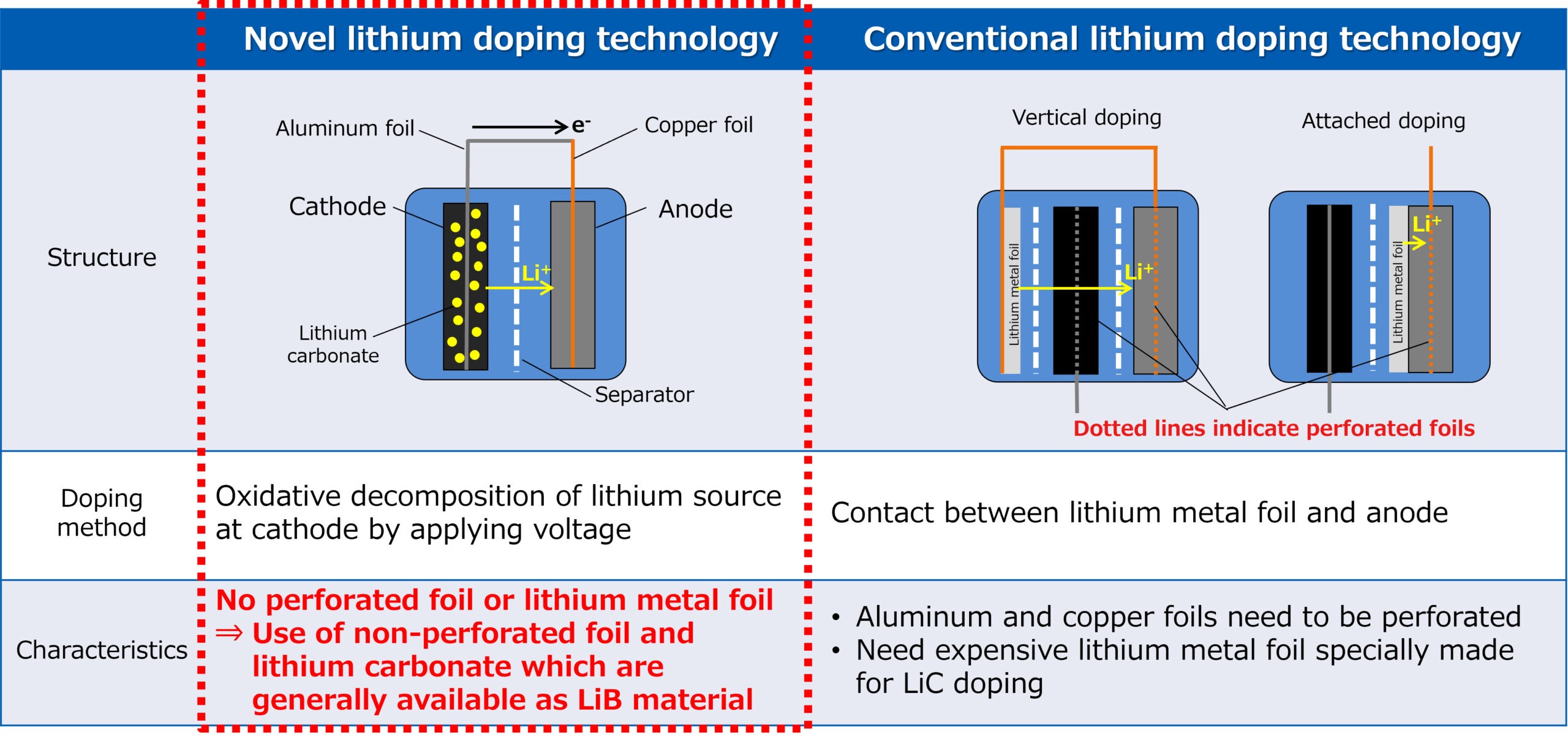
Electrochemistry, a branch of chemistry that deals with the relationship between electrical energy and chemical changes, plays a pivotal role in modern technology. From energy storage systems to medical devices and corrosion protection, the principles of electrochemistry are integral to numerous technological advancements. This 1000-word article explores the various applications of electrochemistry in modern technology, highlighting its significance and the potential future developments.
Introduction
Electrochemistry is at the heart of many technological innovations that have transformed our lives. It is the science that bridges electrons and ions, electricity and chemistry, thus finding applications in a variety of fields. Understanding the role of electrochemistry in modern technology not only highlights its importance but also sheds light on potential areas of future development.
Fundamentals of Electrochemistry
Basic Principles
Electrochemistry involves the study of chemical processes that cause electrons to move. This movement of electrons, or electricity, is harnessed in a multitude of ways. Key concepts include oxidation-reduction (redox) reactions, electrode potentials, and the electrochemical cell.
The Electrochemical Cell
An electrochemical cell is the basic unit in electrochemistry, consisting of two electrodes (anode and cathode) and an electrolyte. It is the foundation for batteries, fuel cells, and electrolysis processes.
Electrochemistry in Energy Storage and Conversion
Batteries
Batteries, from small-scale devices in smartphones to large-scale applications in electric vehicles (EVs), are based on electrochemical reactions. Advances in electrochemistry have led to the development of lithium-ion batteries, which offer high energy density, longer lifespans, and are more environmentally friendly compared to traditional lead-acid or nickel-cadmium batteries.
Fuel Cells
Fuel cells, particularly hydrogen fuel cells, are another application of electrochemistry in energy conversion. They offer a clean energy solution by converting chemical energy into electrical energy, with water and heat as the only byproducts.
Electrochemistry in Environmental Applications
Water Purification
Electrochemistry plays a crucial role in water treatment and purification processes. Electrocoagulation and electroflotation are techniques used to remove contaminants from water, making it safe for consumption or release into the environment.
Air Pollution Control
Electrochemical processes are also used in controlling air pollution. Electrostatic precipitators, for instance, use electrical charges to remove particulate matter from industrial emissions.
Medical Applications of Electrochemistry
Biosensors
Electrochemical biosensors are widely used for medical diagnostics. They are used to detect and quantify biological or chemical components in blood, saliva, or urine. Glucose meters used by diabetic patients are a common example.
Drug Delivery Systems
Electrochemistry is also instrumental in developing controlled drug delivery systems. Electrochemical reactions can be utilized to release drugs at controlled rates, improving the efficacy of treatment.
Corrosion Protection
Cathodic Protection
Electrochemistry is vital in protecting metal structures from corrosion. Cathodic protection, for example, is a technique used to prevent corrosion in pipelines, ships, and steel-reinforced concrete.
Coatings and Paints
Electrochemically created coatings and paints are another method for protecting materials from corrosion, wear, and tear.
Electrochemistry in Electronics and Computing
Semiconductor Processing
Electrochemistry is fundamental in the manufacturing of semiconductors. Techniques such as electroplating and etching are crucial in the fabrication of electronic chips.
Energy-Efficient Lighting
Electrochemical processes are used in producing energy-efficient lighting solutions like light-emitting diodes (LEDs).
Analytical Applications
Electroanalytical Techniques
Electroanalytical techniques are essential tools in environmental monitoring, food safety, pharmaceutical analysis, and more. They provide a means to detect and quantify substances through their electrochemical properties.
The Role of Electrochemistry in Nanotechnology
Nanomaterial Synthesis
Electrochemistry is used in synthesizing nanomaterials, which have applications in catalysis, energy storage, and medical devices. Electrochemical methods offer control over the size and shape of nanoparticles.
Challenges and Future Directions
Advancing Battery Technology
One of the primary challenges in electrochemistry is developing more efficient, sustainable, and safer battery technologies. Research is focused on improving battery capacities, charging rates, and developing alternative battery chemistries.
Hydrogen Economy
Electrochemistry is central to the vision of a hydrogen economy. Developing efficient and cost-effective methods for hydrogen production, storage, and fuel cell technologies is a significant area of research.
Environmental Sustainability
Electrochemistry plays a critical role in developing sustainable technologies. From green synthesis methods to renewable energy technologies, electrochemistry is key in reducing environmental footprints.
Conclusion
The role of electrochemistry in modern technology is vast and multifaceted. From powering our devices to protecting the environment, and advancing medical technologies, the contributions of electrochemistry are integral to our daily lives. As we face global challenges like climate change and resource depletion, the importance of danatoto