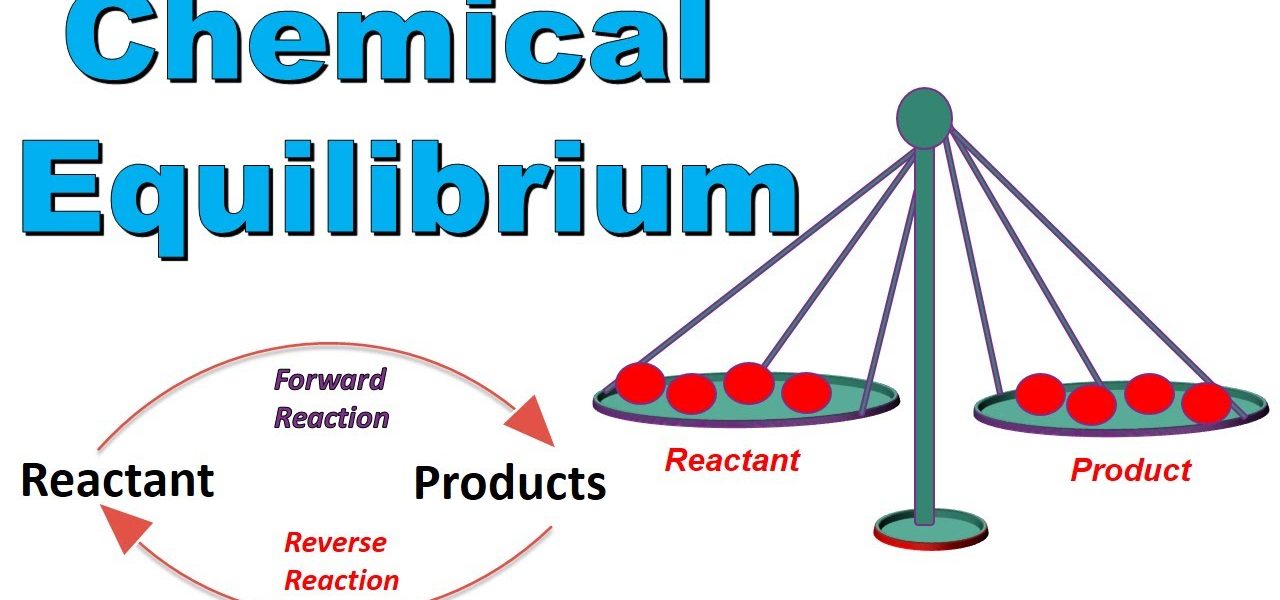
In the ever-evolving world of chemistry, certain concepts stand as fundamental cornerstones, and chemical equilibrium is undoubtedly one of them. At a glance, it appears as a static state where reactions have halted. However, delve deeper, and you’ll discover a dynamic dance of molecules, perpetually in motion. This article explores the marvel of danatoto chemical equilibrium, its significance, and its application in the world around us.
A Balanced Act: Understanding Chemical Equilibrium
At its core, chemical equilibrium refers to a state in which the rate of the forward reaction equals the rate of the reverse reaction. Contrary to popular belief, the reactions haven’t stopped. Instead, they continue at identical rates, leading to no net change in the concentration of reactants and products.
Le Chatelier’s Principle: Nature’s Balancing Act
Introduced by Henry Louis Le Chatelier, this principle states that if a change in conditions is applied to a system at equilibrium, the system will adjust itself to counteract that change. Whether it’s pressure, temperature, or concentration, systems strive to achieve a new equilibrium.
Applications in Everyday Life:
1. Carbonated Beverages: When you uncap a soda bottle, you hear a hiss. This is due to the release of carbon dioxide, a result of a shift in the equilibrium between carbon dioxide in its gaseous form and dissolved form.
2. Blood Oxygen Levels: Hemoglobin in our blood carries oxygen. The equilibrium between oxygenated and deoxygenated hemoglobin plays a crucial role in ensuring our tissues receive adequate oxygen.
Factors Influencing Equilibrium:
1. Concentration: Changing the concentration of either reactants or products will shift the equilibrium, favoring either the forward or reverse reaction.
2. Temperature: Being an essential factor, altering temperature can not only shift the equilibrium but also determine the feasibility of certain reactions.
3. Pressure: Primarily for gaseous reactions, changes in pressure can significantly influence the position of equilibrium.
K_eq: The Equilibrium Constant
This dimensionless value is a measure of the ratio of concentrations of products to reactants at equilibrium. A K_eq greater than one indicates a system where products dominate, while a value less than one suggests the opposite.
Dynamic Yet Constant: A Paradox
Perhaps the most fascinating aspect of chemical equilibrium is its paradoxical nature. While it represents a state of balance and constancy, it’s inherently dynamic. Molecules are in ceaseless motion, reacting and interacting, yet maintaining an overall stable state.
Challenges and Advances:
Modern chemistry continually grapples with manipulating equilibria. Whether it’s in industrial processes aiming for maximum product yield or environmental efforts to shift equilibria and reduce pollutants, understanding and harnessing this principle is pivotal.
Conclusion
Chemical equilibrium, a seemingly simple concept, is profoundly significant in both academic understanding and real-world applications. Its dynamic yet constant nature serves as a testament to the intricacies and wonders of the chemical world.