Chemical equilibrium is a pivotal concept in chemistry, representing a dynamic balance between forward and reverse reactions in a system. This delicate balance is governed by principles elucidated by Le Chatelier and quantified by equilibrium constants. This article embarks on a journey through the world of chemical equilibrium, unraveling the factors that influence equilibrium positions and exploring the dynamic interplay of reactions. From reversible reactions to the effects of concentration changes and temperature variations, we delve into the intricate nature of chemical systems.
1. Dynamic Equilibrium: A State of Balance
Chemical equilibrium is a dynamic state where the rates of forward and reverse reactions are equal, resulting in no net change in concentrations.
2. Le Chatelier’s Principle: Shifting the Balance
Le Chatelier’s principle states that a system at equilibrium will respond to changes in conditions by shifting the position of the equilibrium to counteract the change.
3. Equilibrium Constant (Kc): Quantifying Balance
The equilibrium constant expresses the ratio of concentrations of products to reactants at equilibrium, providing valuable information about the extent of a reaction.
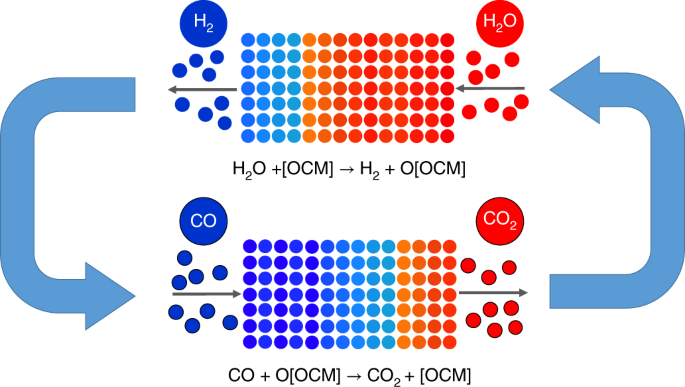
4. Reversible Reactions: Forward and Back Again
Reversible reactions can proceed in both the forward and reverse directions, allowing for the establishment of equilibrium.
5. Equilibrium Position: Finding the Sweet Spot
The equilibrium position refers to the relative concentrations of reactants and products at equilibrium, which can be influenced by external factors.
6. Concentration Changes: Disturbing the Peace
Changing the concentrations of reactants or products in an equilibrium system prompts a shift in the equilibrium position to restore balance.
7. Pressure Changes: Affecting Gas-Phase Equilibria
For reactions involving gases, changes in pressure can influence the equilibrium position, particularly in systems with differing numbers of gas molecules.
8. Temperature Effects: Heat in the Equation
Temperature alterations can significantly impact equilibrium, with exothermic and endothermic reactions responding differently to changes in heat.
9. Solubility Equilibria: Dissolution Dynamics
In solubility equilibria, sparingly soluble compounds reach a dynamic balance between dissolved and undissolved states.
10. Acid-Base Equilibria: The Dance of Protons
Acid-base equilibria involve the transfer of protons between species, resulting in the establishment of pH levels.
11. Industrial Applications: Harnessing Equilibrium
Understanding and manipulating chemical equilibrium is crucial in industrial processes, from chemical manufacturing to environmental control.
In Conclusion: The Art of Balance
Chemical equilibrium is akin to a delicate dance, where molecules move forward and backward, seeking a harmonious balance. Le Chatelier’s principle and equilibrium constants guide our understanding of this dynamic interplay. By comprehending the factors that influence equilibrium positions, we gain the power to manipulate chemical systems, enabling advancements across industries and scientific disciplines.
In Conclusion:
Chemical equilibrium is akin to a delicate dance, where molecules move forward and backward, seeking a harmonious balance. Le Chatelier’s principle and equilibrium constants guide our understanding of this dynamic interplay. By comprehending the factors that influence equilibrium positions, we gain the power to manipulate chemical systems, enabling advancements across industries and scientific disciplines.